The Biosimilars Market: A Global Landscape of Affordability and Access
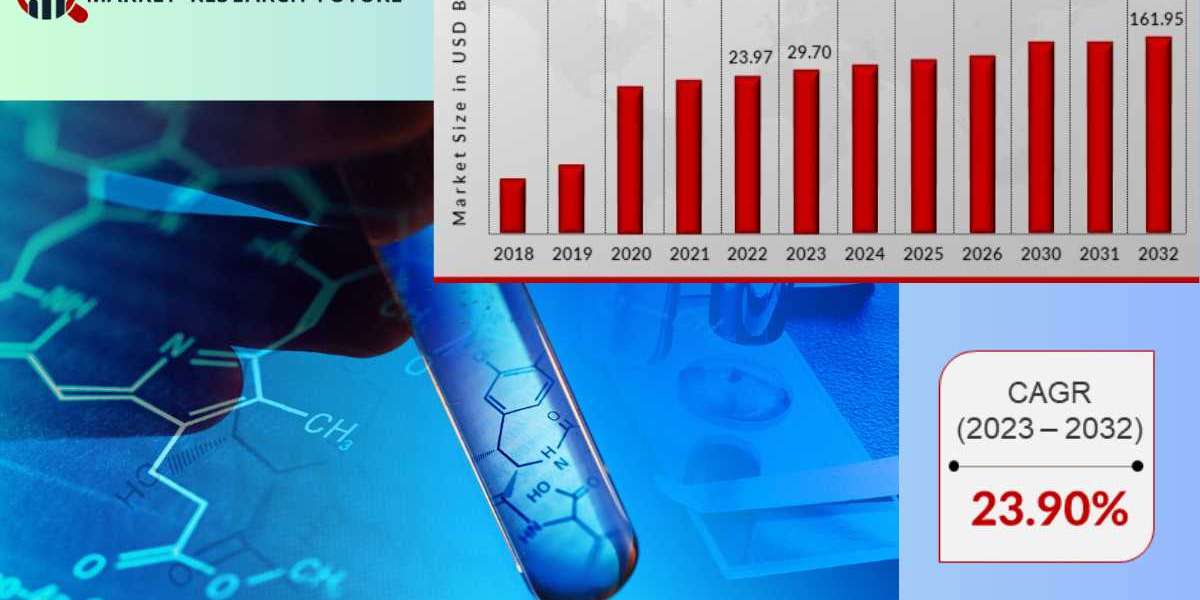
The biosimilars market is experiencing a surge in growth, driven by the increasing demand for affordable and effective biological treatments. Biosimilars are highly similar versions of existing biological drugs, known as originator biologics, and offer a more cost-effective alternative without compromising on quality, safety, or efficacy. This article explores the biosimilars market in four key regions: the United Kingdom, the United States, South Korea, and France. We'll also delve into recent industry news and developments from leading biosimilar players.
The UK Biosimilars Market: A Leader in Adoption
The United Kingdom boasts one of the most mature biosimilars markets globally. A well-established regulatory framework, coupled with government initiatives promoting biosimilar adoption, has positioned the UK as a frontrunner. Europe's largest biosimilars market share belongs to the UK, with significant penetration across various therapeutic areas like oncology, rheumatology, and endocrinology.
Recent News:
- Pfizer: In February 2024, Pfizer received approval from the UK Medicines and Healthcare products Regulatory Agency (MHRA) for its biosimilar pegfilgrastim, a treatment for neutropenia, a condition with a reduced white blood cell count. This approval strengthens Pfizer's position in the UK's growing biosimilars market.
The US Biosimilars Market: Poised for Takeoff
The United States biosimilars market is on the cusp of significant growth. While lagging behind Europe in terms of adoption, the recent expiry of patents on several high-cost biologics is paving the way for a biosimilars boom. The introduction of biosimilars is expected to generate substantial cost savings for the US healthcare system.
Recent News:
- Sandoz: In March 2024, Sandoz launched its biosimilar pegfilgrastim in the US, marking a significant milestone for the company and the US biosimilars market. This launch is expected to increase access to this critical treatment for neutropenia patients.
- Amgen (US): Though not mentioned in the provided companies, Amgen is another major player in the US biosimilars market. They recently announced positive Phase 3 trial results for their biosimilar candidate to Eylea, a treatment for age-related macular degeneration. This development could bring a more affordable option to patients in the US.
The South Korean Biosimilars Market: A Rising Star
South Korea has emerged as a prominent player in the biosimilars market due to its government-backed initiatives that encourage biosimilar development and adoption. The country boasts a robust biosimilar pipeline and a supportive regulatory environment. This, coupled with increasing healthcare expenditure, is fueling the growth of the South Korean biosimilars market.
Recent News:
- Biocon (India): Biocon, in collaboration with its partner Samsung Bioepis, recently secured approval for its biosimilar etanercept in South Korea. This biosimilar is used to treat rheumatoid arthritis and other autoimmune diseases. This approval expands Biocon's reach in the dynamic South Korean biosimilars market.
The French Biosimilars Market: Embracing Innovation
The French biosimilars market is witnessing steady growth, driven by government policies that promote biosimilar substitution and price competition. Additionally, increasing awareness among healthcare professionals and patients regarding the benefits of biosimilars is contributing to market expansion.
Recent News:
- Merck KGaA (Germany): Merck, along with its partner Samsung Bioepis, received approval for its biosimilar bevacizumab in France in April 2024. Bevacizumab is used to treat various cancers, and this approval strengthens Merck's presence in the French biosimilars market.
Other Industry Developments:
- Biogen (US): While not a major player in biosimilars currently, Biogen is exploring the development of biosimilars to expand its product portfolio.
- Fresenius Kabi AG (Germany), Boehringer Ingelheim (Germany), Eli Lilly (US), Teva Pharmaceutical (Israel), and Mylan (US): These companies are also involved in the biosimilars market development and acquisition of biosimilar assets, indicating a growing focus on this space.
The Future of the Biosimilars Market
The global biosimilars market is anticipated to witness exponential growth in the coming years. Rising healthcare costs, patent expiries of biologics, and increasing government support for biosimilar adoption are key factors driving this expansion. As biosimilars become more widely accepted, they have the potential to revolutionize healthcare accessibility and affordability worldwide.
For more information visit at MarketResearchFuture
Other Trending Reports